Windscreen washer fluids (ScreenWash) - frequently asked questions
We will try to answer in this article some of the frequently asked questions to the quality of windscreen washer fluids (windshield wiper fluid, wiper fluid, screen wash, washer fluid).
The basis for this article, several papers published by Ing. Jan Skolil, head of quality and development in company CLASSIC Oil s.r.o., the leading producer of windscreen washer fluids in the Czech Republic. We would like to thank him for the kind permission to use his work and also for the final review of this text.
I bought the windscreen washer fluid declared as -40 °C, but by using a refractometer I measured only -27 °C
Determination of freezing point using a refractometer is an indirect method of measurement. We do not measure directly the freezing point, in the fact we measure refractive index (see Snell's law). Because we know the dependence of the refractive index on the alcohol/water mixture concentration and we know also the dependence of freezing point on that concentration, we are able to determine the freezing point (temperature of solidification) of mixtures by using a refractometer.
A windscreen washer fluid contains water, main antifreeze ingredient (an alcohol) and the additional ingredients (polyhydric alcohols such as ethylene glycol, propylene glycol or Glycerin etc.). The main antifreeze alcoholic ingredient is usually one of the alcohols, which we will continue to talk – Isopropyl alcohol, Ethanol or toxic Methanol. Due to the costs and availability is in Europe mostly used Ethanol. Watch out, because the Isopropanol + water mixture and Ethanol + water mixture have completely different dependence of the refractive index on their concentrations!
Therefore it is necessary to know not only which substance we measure but also which device we should to use. It is necessary to select the correct refractometer to be not unpleasantly surprised by the measurement results. There are several refractometers with different scales designed for measuring of windscreen washer fluids. Unfortunately there are widely used refractometers which have just one scale for for measuring of windscreen washer fluids. Although it is nowhere expressly declared the scale is mostly calibrated on a Isopropanol + water mixture. And that is why sometimes are published erroneous results when someone unaware of this issue try to make a survey of mixtures available on the market.
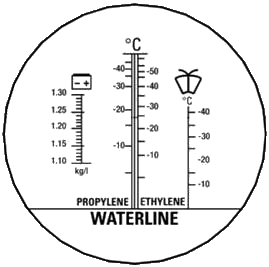
As an example of the use of inappropriate refractometer I bring the following: if we measure a Ethanol + water mixture using a refractometer designed for Isopropanol + water mixture (see the picture on the left) can we get completely misleading results! At -20 °C have both mixtures approximately the same index of refraction. But when the scale designed for Ethanol + water mixture shows -40 °C the scale designed for Isopropanol + water mixture shows only -25 °C!
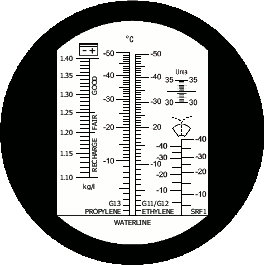
Refractometer RBC4AB-ATC is equipped with two scales for measuring of windscreen washer fluids, two scales for measuring of engine coolants (there are the same problems when you measure engine coolants), scale for measuring of battery electrolyte condition and scale for measuring of liquid additive AdBlue®. You can see the scales of this refractometer on the second picture on the right: scales for windscreen washer fluids are under the „wipers icon“ – the scale on the left is designed for Ethanol + water mixture, the scale on the right (signed as SRF1) is designed for mixture Isopropanol + water.
| Refractometer for measuring of windscreen washer fluids (scales under the „wipers icon“) | |
 |
 |
| „PROBLEMATIC“ equipped with only one scale designed for mixture Isopropanol + water (models sold as RH 403 or REF 404) |
„VERSATILE“ equipped with two scales - on the left Ethanol + water mixture, on the right Isopropanol + water mixture (model RBC4AB-ATC) |
Windscreen washer fluid freezes on my windshield
Aside from a few other possible but unlikely causes of the problem (for example badly mixed mixture with a high water content), there are two main causes of liquid freezing which usually interact:
- Heat dissipation from the windshield – it is widely known, and everybody experiences it firsthand, that the perception of cold is more intense when the wind blows. Flowing air speeds up the heat dissipation from the warmer subject – the temperature of ambient air and the temperature of warmer subject are equalizing. The rate of cooling is proportional to the flow velocity – the faster the wind blows (the faster you go), the faster the temperatures equal.
- Ethanol's high volatility – as already mentioned the base antifreeze ingredient used for windscreen washer fluids is Ethanol (at least in Europe). Ethanol has lower freezing point than water but it is also flammable and volatile. Vapour pressure of water at normal temperature is 2330 Pa, while the vapour pressure of Ethanol is 5600 Pa. It follows from this that the Ethanol produces approximately 2.5 times more vapour than water. Therefore Ethanol also faster disappears from the windscreen surface. At a higher speed is the difference in volatility even more significant.
It follows from the text above that at higher speeds the Ethanol evaporates quickly, the temperature of the windscreen quickly drops to ambient temperature and if the temperature is around 4 °C there will be formed ice crystals at the windscreen. And even if you have a new winter mixture in your windscreen washer system. In the climatic conditions of Central Europe, we recommend to use a mixture with a high percentage of Ethanol and with the freezing point at -30 °C and low (-40 °C etc.). But even such mixture may not be enough when interacts a big freeze and a high speed.
I added a winter windscreen washer fluid (screenwash) concentrate and it froze over night
The modern design of the washer fluid storage tank usually does not allow the simple mixing of the old and new fillings, since it is basically a long narrow tube, where you can't even see what is located at its bottom. Due to the fact that Ethanol is mixed with water just on the basis of hydrogen bonds and the efficiency decreases with decreasing temperature. It is therefore not surprising that the concentrate does not react even a few hours with the frozen water in the tank and does not melt the ice. Furthermore, it is necessary to note, the Ethanol density is lower than density of the water (less than 820 kg/m3) and therefore Ethanol floats on the water surface. So the mixing is not supported even by gravity. Therefore it can easily happen that the residual summer washer fluid froze in the circuit during the night although we added a winter concentrate at the evening.
The explanation is simple – the lower layer (containing mostly water) froze, while the upper layer (mostly Ethanol) is liquid (a small "liaison" area in the narrow tube prevents the required mixing water with Ethanol).
As a precaution it is sufficient to use a winter washer fluid all year or to replace the summer washer fluid before the winter season by a winter washer fluid. Summer fluid should be completely sucked out and only then should be the washer fluid circuit filled with a winter fluid.
Washer fluid etched my headlights
Ethanol is in Europe produced usually from grain, sugar beet or potatoes, Ethanol imported from overseas is produced often from sugar cane or maize (corn). Due to possible tax evasion must be all Ethanol for technical use denatured. And that is the problem! There is a lot of denaturing agents - official as well as specially permitted. The common, such as (Ethyleneglycol, Isopropanol, Bitrex/Denatonium Benzoate) are established and do not harm any part on the car.
On the contrary, the less official, but often cheaper denaturing agents (for example, Acetone, Methanol, Ketone, Acetaldehyde) can etch the headlights. Polycarbonate (PC) or Polymethyl methacrylate (PMMA), which are used as the main structural material of the vehicles headlights, are more prone to the etching by these substances.
Headlights material belongs among the most durable transparent polymers becuase it is exposed to the harshest treatment (exposure to light and UV radiation, thermal load, road salt influence, washing by a washer fluids etc.). The damage of the headlights is not usually visible immediately. So if you previously used the bad washer fluid, the damage may occur even you already use a quality washer fluid with pure Ethanol.
Unfortunately, not all washer fluids contain only pure Ethanol. May be supplemented by substances which are recognizable by smell (Acetone, Acetaldehyde), but also by recognizable substances (Methanol, some Ketones etc.).
The conclusion
Use year-round only proven and branded washer fluids - both summer and especially winter mixtures. Such, whose producers have long-term experience, does not experiment with the fluid composition and use only high quality, purified, and appropriately denatured European ethanol.
The information in this article gives an advice for choosing the right washer fluid and prevents spreading of the various rumors and "trusted" information among the motorists.